This ECG is from a 66-year-old woman who called 911 for a complaint of chest pain for the past four hours. She also complained of nausea, vomiting, and diarrhea for that time. She was pale and diaphoretic, and her BP was 77/43 sitting up, improving to 90/54 reclining. She denied “cardiac” history. Her medications included: aspirin, an SSRI, cilostazol, amlodipine, umeclidinium and vilanterol inhaler, atorvastatin, levothyroid, and metoprolol. We don’t have a previous ECG. The EMS crew followed their chest pain protocol and delivered the patient to a facility with an interventional cath lab, but they did not designate a “STEMI Alert” because of the wide QRS. It is their protocol to use the term “STEMI Alert” only when no M.I. mimics, such as left bundle branch block, are present.
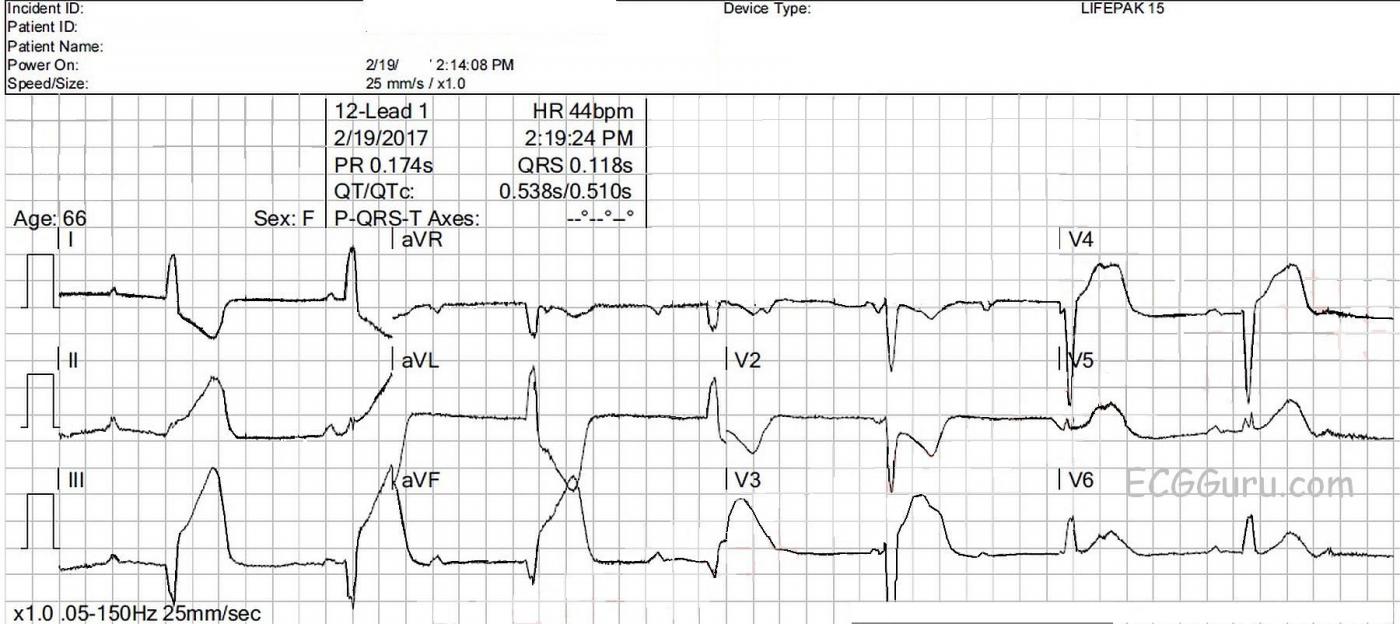
What does this ECG show? There is an underlying sinus rhythm at 75 bpm. There is AV dissociation, with regular, wide QRS complexes at a rate of 44 bpm. This meets the criteria for complete heart block (third-degree AV block). The morphology of the QRS complexes meets the criteria for left bundle branch block (wide, upright in Leads I and V6, negative in V1). At a rate of 44 bpm, several options for this escape rhythm are possible: 1) junctional escape with LBBB, 2) junctional escape with intraventricular conduction delay due to AMI, and 3) idioventricular escape rhythm. Also, in the presence of IWMI, AV node ischemia is very likely, resulting in AV blocks at the level of the AV node. CHB at the AV node would result in junctional escape rhythm, and CHB below that, in the fascicles of the bundle branches, would result in idioventricular escape. The issue for this patient, and ANY patient, is cardiac output, and we see several reasons for cardiac output to be lower:
· Wide QRS
· Slow rate
· Lack of P waves preceding every QRS (loss of atrial kick).
In the EMS setting, it really doesn’t matter if the escape rhythm is junctional with wide QRS or ventricular. The patient's hemodynamic status is the important consideration.
Even more alarming, this ECG shows signs of acute inferior wall M.I. It can be difficult to ascertain when STEMI is present in the presence of wide-complex rhythms. That is because most wide-complex rhythms have discordant ST and T wave changes. That is, whenever the wide QRS is positive, there is ST depression and T wave inversion, and whenever the wide QRS is negative, there is ST elevation and upright T waves.
This ECG shows excessively elevated discordant ST segments in the inferior leads (II, III, and aVF.) We also see excessively discordant ST elevation in V3, and V4. The change from ST depression to ST elevation between V2 and V3 is very abrupt, with the obvious ST depression in V1 and V2 indicating reciprocal views of ST elevation on the posterior wall. In LBBB without STEMI, there is normally ST elevation in V1 a V3.
Sgarbossa and Smith In 1996, Sgarbossa, et al proposed a univariate scoring system for determining acute M.I. in the presence of LBBB. Sgarbossa’s Criteria has been used for with some success both in the presence of LBBB and ventricular paced rhythms. These criteria were formulated before results could be confirmed with cath lab results. In this decade, Dr. Steven Smith and his colleagues have proposed some modifications to Sgarbossa’s Criteria which take into account the ratio of ST alteration to R wave. In Smith’s Modification, excessive discordance is measured as discordant ST elevation when the j point is > 0.25, or 25% the depth of the S wave. His results have been, and continue to be, measured against cath lab findings, and are more accurate than the original criteria. For an excellent discussion of LBBB, Sgarbossa’s Criteria, and Smith’s modified Sgarbossa criteria, we recommend Tom’s Bouthillet’s excellent three-part series on the topic.
With the exception of right bundle branch block, most wide-QRS conditions are considered “mimics” of acute M.I., and can both disguise the presence of an M.I. and masquerade as M.I. Unfortunately, the mimics do not prevent the patient from having an M.I.
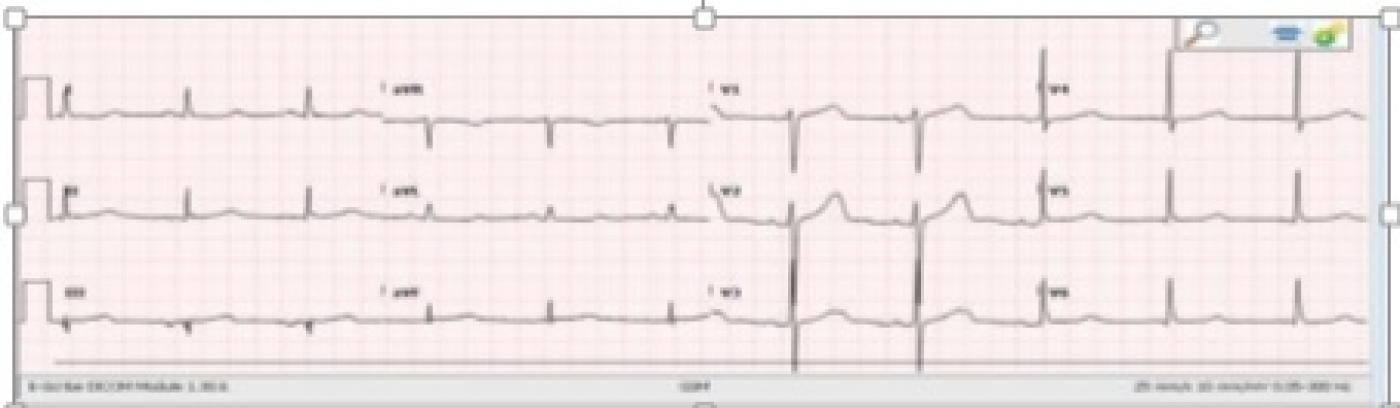
How did this patient do? The infero-lateral M.I. was recognized in the emergency department, and the patient’s hypotension was treated with pacing and fluids. She was sent immediately to the cath lab, where it was found that she had a single-vessel lesion in the proximal to mid right coronary artery. There was 100% occlusion with TIMI-0 flow. She underwent angioplasty and stent placement, with excellent TIMI-III results. The RCA was dominant, and much larger than the LCA. The second ECG shows the excellent results of the angioplasty - QRS is narrow, the rhythm is sinus, and ST segments returning to normal. The tiny Q wave in Lead III eventually disappeared, probably because it was due to right ventricular M.I.
This crew felt they were following their protocol in not calling this a “STEMI Alert”, but fortunately they were able to transport the patient to a full-service cardiac hospital, where she received angioplasty very quickly.
All our content is FREE & COPYRIGHT FREE for non-commercial use
Please be courteous and leave any watermark or author attribution on content you reproduce.


Comments
STEMI Alert Indicated Despite Wide QRS ...
Interesting case and excellent discussion by Dawn. I’ll add the following points.
i) The RHYTHM — Use of CALIPERS can be invaluable for rapid assessment of complex arrhythmias. Many of the P waves on this tracing are hidden within the huge QRST complexes. Despite this, setting your calipers to the P-P interval for the P wave just before and just after the 2nd beat in lead (best seen in leads III,aVF) allows you to rapidly confirm regularity of the P-P interval. And since the R-R interval is also quite regular, but with no relation between P waves and neighboring QRS complexes — you have established the diagnosis of 3rd-Degree ( = Complete) AV Block with a ventricular escape rate between 45-50/minute. QRS morphology is highly characteristic of complete LBBB — which as per Dawn suggests AV Nodal Escape with LBBB rather than an accelerated ventricular escape.
ii) It would have been nice to have a long-lead rhythm strip at the bottom of the tracing. Instead, all we have is a 12-lead. That said, leads are continuous — so we can still make the diagnosis of complete AV block (as per i).
iii) Although modified Smith-Sgarbossa criteria may be helpful with assessment of ST-T wave changes in the presence of LBBB when there is inappropriate discordance — there is NO NEED to invoke these criteria in this tracing! That’s because MANY leads clearly manifest flagrant primary ST-T wave changes that are obvious regardless of whether the rhythm here is junctional escape with LBBB or ventricular escape. That is, the shape and amount of ST elevation/T wave peaking is clearly diagnostic of acute stemi in each of the inferior leads. ST-T waves manifest obvious hyperacute changes in leads V3-thru-V6 — and, shape of ST depression in leads I, aVL and V2 is clearly abnormal. With experience, recognition of the magnitude of ST-T abnormality in each of the above leads in a patient with new chest pain, should allow instant diagnosis of acute STEMI. So while understood that certain EMS system may establish rules such as exclusion of cath lab activation when there is QRS widening — targeted training with regular feedback and oversight will hopefully lead to an increasing number of EMS systems advancing to the point where immediate cath lab activation is both allowed and encouraged despite QRS widening when obvious criteria are met.
iv) This case is a superb illustration of obvious acute STEMI despite the presence of LBBB.
v) Prediction of the “culprit artery” is indeed challenging in this case. But retrospectively, I would say that ALL the clues are present to suggest acute RCA ( = Right Coronary Artery) occlusion. These are: i) Bradycardia and hypotension; ii) Maximal ST elevation in the inferior leads, with ST elevation in lead III > lead II, with dramatic ST depression in reciprocal lead aVL + evidence (in leads V1,V2) of associated posterior involvement. One may see lateral chest lead ST elevation with acute RCA occlusion (as we do here) IF there are large posterolateral branches off the PDA (posterior descending artery) — albeit one usually doesn’t see quite so much ST elevation in V3,V4 with that lesion as we do here.
vi) Atropine could have been used to treat excessive bradycardia/hypotension (IF the patient was symptomatic) — since recent onset of acute inferior stemi with bradycardia/AV block may often respond to prompt treatment of excess vasovagal tone.
Ken Grauer, MD www.kg-ekgpress.com [email protected]
An Excellent Teaching Case!
Dawn...
Thank you for a very thorough and expert interpretation. I want to show this ECG, followed by your discussion, to some of my colleagues in internal medicine and emergency medicine to see their reaction. In my teaching, I have found that so many providers of medical care seriously overestimate their knowledge and skill in interpreting ECGs. I'll bet that both you and Dr. Grauer have noticed this tendency also. And thanks to Dr. Grauer for his excellent additional comments.
I do want to discuss something else about this ECG. Dr. Grauer hinted at it, but I want to take it further. On this single ECG we have a diagnosis of acute inferior epicardial ischemia (II, III and aVF) and acute lateral epicardial ischemia (V5 and V6) as you pointed out and posterior (now we should call it "lateral") epicardial ischemia as Dr. Grauer indicated. This is manifested by the downward-sloping ST segments and the inverted T waves in V1 and V2 (yes, T waves are initially inverted in posterior MIs). So we have accounted for the effects of a proximal RCA occlusion in precordial leads V1, V2, V5 and V6. This is not that unusual - a posterior ("lateral") MI often accompanies an inferior MI whether it is an occlusion of the RCA or the LCx (in this case, we know it's the RCA). And, as Dr. Grauer pointed out, sizable posterolateral branches from the RCA can also result in an infarct area in the lower lateral LV covered by V5 and V6. Again, not that unusual. But I have three questions:
1) The lower lateral area of the left ventricle is usually supplied by branches of the LAD, the LCx and occasionally the ramus intermedius in addition to the RCA. Yet, in this case, a single occlusion of the RCA infarcted (or at least made "acutely ischemic") that normally well-perfused area. Why?
2) There is unquestionably an acute posterior epicardial ischemia present, but there is very minimal ST or J-point depression in V1. In fact, if there were no other abnormal changes on the ECG, I would be tempted to call V1 normal. Why is there not more ST depression in V1?
3) Why do we have ST elevation in V3 and V4? These areas of the anterior left ventricle are not covered by the RCA. Why is there ST elevation in V3 and V4?
Here is what I think...
ST elevation reflects an injury vector. The injury vector points TOWARD the leads overlying the acutely ischemic area. For there to be a positive injury vector in all three inferior leads - II, III and aVF - the injury vector must be pointing somewhere between +30 degrees and +150 degrees on the hexaxial reference grid. Therefore, the reciprocal to the injury vector (the opposite end) must be pointing somewhere between -30 degrees and -150 degrees. For both Lead I and aVL to manifest reciprocal changes (negative ST-T segments), they must be pointing between -120 degrees and -150 degrees. If the negative end of the injury vector were pointing between -120 degrees and -90 degrees, it would be negative (as excpected) in Lead I but it would fall within the positive hemisphere of aVL, meaning aVL would not show any ST depression.
I am also very impressed by the depth of the reciprocal change in Lead I. Lead I is indicating a reciprocal change (i.e., the "negative" end of the injury vector) to something that is much further to the right - namely, the RIGHT VENTRICLE. In the presence of an inferior STEMI, always be wary of ST depression in Lead I. Look at a lot of acute inferior STEMIs - reciprocal changes in Lead I are usually not there or, if present, are very subtle. Lead aVL usually shows the reciprocal changes (and those are to Lead III, mostly). So I believe there is a right ventricular MI occurring also. Did you notice that the physicians in the ED treated the patient with FLUIDS in addition to a (I assume external) pacemaker? Patients with just an inferior MI are not fluid-depleted. All that is usually necessary to improve their hemodynamic status is to get their heart rate up, which can be done with atropine or isoproterenol. But this patient was treated with fluids! During an acute right ventricular infarction, the right venticle basically becomes a minimally-effective conduit and the patient's pressure is maintained by increasing the return to the left atrium and that is done by administering fluids.
This is why there are such minimal changes in V1 - the STE of RV ischemia is counteracted by the reciprocal ST depression of a posterior STEMI. It also explains the changes in V3 and V4. Those STE's aren't reflecting acute epicardial ischemia of the anterior wall of the LV - they're reflecting STE of the anterior wall of the RV. I'm not 100% sure why there is as much ST depression in V2 as there is, but I would suspect that there might have been a lot more, possibly with a more flattened ST segment, if there were no right ventricular infarct present.
The answer to the first question provides the reason for the issues in the other two questions: I think this was a "superdominant" RCA. There was probably no ramus intermedius present since they are absent in most people. Either the other two arteries were small, or they had distal disease and the RCA was providing collateral circulation. The fact that the RCA caused the STE in V3 and V4 indicates that it was providing circulation to the anterior wall of the right ventricle - increasing the likelihood that this represents a superdominant RCA. Most people don't know this, but the LAD is the normal major blood supply to the anterior wall of the right ventricle - yes, I said LAD and I also said RIGHT ventricle! The right ventricular (acute marginal) arteries mostly provide circulation to the posterior and lateral wall of the RV.
If you other website visitors would like to ask questions of Dawn, Dr. Grauer or myself regarding one of these ECGs, please submit them to this website so we can start a dialogue. Everyone will benefit. And if you are an ECG newbie - GREAT! You are most welcome to ask basic questions or submit your own ideas about what is going on. This is a website for people to LEARN about electrocardiography and all the more advanced ECG interpreters here want to ENCOURAGE you. "Stupid" questions are especially solicited because until they are correctly answered, they will never go away. In my advanced ECG classes I always begin by telling my students: "Now is a great time to ask all those really, really STUPID questions that have been bothering you... because when you leave here at the end of the course, you will never have to suffer the humiliation of seeing any of these people again!"
Thanks for a great case and a phenomenal website. Hope everything went well in Florida!
Jerry W. Jones, MD FACEP FAAEM
Jerry W. Jones MD FACEP FAAEM
https://www.medicusofhouston.com
Twitter: @jwjmd
Thank You Dr. Jones!
Great comments by Dr. Jones on this tracing! I contemplated mentioning acute RV involvement in my original comment, because of the relatively modest amount of ST depression in lead V1 — and also contemplated postulating the RCA as also providing circulation to the anterior wall of the RV as the explanation for that unexpected ST elevation in leads V3,V4 — since I don't otherwise know how to explain all findings that we see here from a single "culprit" artery. So it IS so VERY helpful here that we KNOW from Dawn that there indeed was a single culprit RCA. However, I didn't think I had enough firm evidence to postulate all of that ... Credit to the convincing discussion by Dr. Jones, that I agree really provides the ONLY way I can conceive of a single culprit artery in this case. THANK YOU Jerry for adding so much to this discussion!
Ken Grauer, MD www.kg-ekgpress.com [email protected]
Thank you, Drs. Grauer and Jones!
Your comments are so much appreciated. This is such a great teaching case. I almost mentioned the RVMI in my initial comments, because of the discrepency between the ST depression in V1 and V2, Lead III STE > than Lead II STE, and the ST changes in Lead I. But, for some reason (I think I felt I was getting to long-winded), I forgot to include it. Now, I am glad I did because the comments from both of you regarding culprit artery and the almost definite presence of RVMI were so much more instructive than what I would have written.
On our FaceBook page, a controversy arose regarding the conduction delay, and whether or not to call it "LBBB". Dr. Grauer fielded this one very well. I want to say that, because we do not have EP studies on the patient, and because "LBBB" can mean several different conditions, I did not think it was more than a semantics issue. The QRS is wide, but there is a definite IWMI. That is what is actually important to the patient. I personally don't think Sgarbossa's or Smith's criteria are needed here, but whenever I post a wide QRS with an M.I., I get asked about it. Many of the paramedics teach are not permitted to designate a patient with a wide complex (with the exception of RBBB) as a STEMI Alert. I suppose this crew could have circumvented that rule because the QRS was less than .12 sec. However, they did not recognize the presence of the M.I. They did correctly determine that the patient was suffering from a cardiac emergency, and delivered her to a full-service cardiac hospital, where she underwent successful angioplasty.
Thanks again for the stimulating conversation, and for the comments Dr. Jones made regarding questions. BY ALL MEANS, if you have a question, you will get a good answer from one or all of us, and you are anonymous! So great to finally know the answer about something you haven't understood in the past. I love that feeling! Please comment and question!
Dawn Altman, Admin
About the Wide QRS Complexes
First, I do want to say that the paramedics followed their protocol and this patient had an excellent outcome. I wonder if this ECG was transmitted to a medical control physician who should have been able to diagnose an acute inferior MI at the very least. Also, the ECG print-out gives the QRS duration as less than .12 seconds, so technically this isn't LBBB. However, I seriously doubt that I could have determined that the QRS was 0.11 seconds (and change) by looking at it, so I would have called it LBBB.
There is too much misunderstanding about LBBB and acute infarctions with a pervasive concept that is it extremely difficult - even with the modified Smith-Sgarbossa criteria - to diagnose an acute MI when LBBB is present. This problem reminds me of a physician I used to know. If she detected even the slightest hint of an accent in anyone's speech she would immediately proclaim "I can't understand a word he's saying. Someone talk to him and tell me what he's saying!" Just as she refused to listen to what the other person was very clearly telling her, so do many medical people throw up their hands and refuse to look at what the ECG with a LBBB is telling them.
The main issue with LBBB and acute MI deals mainly with V1 and V2 and whether an anteroseptal MI is present. It really doesn't affect the other leads so much and it certainly doesn't have any confounding effect on the inferior leads. The original third criterion referred to ST elevation in ANY lead with a predominantly negative QRS complex, but this really only applied to those leads with negative QRS complexes that actually reflected the repolarization abnormality of the LBBB (and that is mainly V1-V2). In this ECG, the inferior leads were negative, but the rule really shouldn't apply to them because the inferior leads do not reflect the repolarization changes of LBBB - even when they are negative.
I think it's about time that we start getting people to pull their head out of the sand regarding LBBB and acute epicardial ischemia and realize that many MIs remain quite obvious - even in people with LBBB! The whole idea of the Sgarbossa criteria was to get physicians and other practiioners to understand that you CAN diagnose MIs in the presence of LBBB. With the Smith modification, the Sgarbossa criteria really become more reliable based on the third criterion. The first and second criteria are basically for diagnosing acute MIs under ANY circumstances - not just LBBB. Unfortunately, the idea that you can't make the diagnosis of an acute MI with LBBB still remains pervasive in the medical field. That doesn't surprise me since it can take 40 years before facts are finally acknowledged in electrocardiography. Look how long it took for us to accept the fact that the heart is NOT in the Valentine position in the chest and that T wave inversion is NOT the first sign of ischemia. We need MORE ECG education in this country (and the rest of the world, too!). I congratulate the paramedics for following their protocol and doing their job well, but I DO have two concerns: one, about a protocol that is probably outdated and two, if the ECG WAS transmitted to a medical control, that the several infarctions weren't recognized before delivery of the patient.
Jerry W. Jones MD FACEP FAAEM
https://www.medicusofhouston.com
Twitter: @jwjmd
Dr. Grauer,
Dr. Grauer,
Would Atropine be helpful for this patient? In the presence of a complete heart block, would Atropine increase the ventricular rate? If so, how? Would this patient, if symptomatic, be better treated with TCP or B-adrenergic support (i.e. epinephrine or dopamine)?
J. Henson
I didn't understand this:
I didn't understand this:
"We also see concordant ST elevation in V3, V4"
Wouldn't any elevation have to be discordant since the QRS is almost completely downward in leads V3 and V4?
Answer to Rod
Thank you! You are right, and the original text was a typo on my part! Sometimes, I think ahead to the next thought before finishing my typing! Thanks to your good eye, I have corrected that part.
I also want to mention that i only cited Sgarvossa and Smith's criteria because so many of my own students have expressed the belief that we CANNOT diagnose STEMI in the presence of wide QRS complexes. Since this is obviously not true in this case, experiences ECG interpreters do not need to turn to the criteria. The suggested reading is very good, however, to those who are not familiar with this concept.
Thanks again!
Dawn Altman, Admin
Answers to Questions by JHenson & Rod
I wanted to address the insightful 2 questions just submitted, which serve to highlight the most important points about this case:
Question #1 (by JHenson): Would Atropine be helpful for this patient? My answer is possibly yes — but it depends! Atropine is a parasympatholytic agent that may be helpful in selected cases of symptomatic bradycardia due to excess parasympathetic tone. The drug may increase the atrial rate, and improve AV nodal conduction. However, it is not a benign agent — since by reducing parasympathetic influence, it may leave underlying sympathetic tone unopposed. This may in certain circumstances produce tachycardia and increase ischemia. It is for this reason that use of this drug should be limited to cases of “symptomatic” bradycardia.
Many patients with acute MI have increases in both sympathetic and parasympathetic tone. As a general rule, increased parasympathetic tone is more likely to predominant with acute inferior infarction — because the most likely “culprit” artery in this case is the RCA, which usually supplies the AV node. This is why acute inferior MIs are often accompanied by bradycardia, especially during the early hours of acute MI. But there is little parasympathetic innervation in the ventricles, so ventricular escape rhythms are much less likely to respond to a parasympatholytic agents such as Atropine.
This case is complicated — because: i) there is complete AV block with a wide QRS escape rhythm; and ii) Other treatments may be better. So, many of the ECG subtleties we described in detailed above ARE relevant to answering Question #1. That is, although QRS widening with complete AV block usually suggests a ventricular site for the escape rhythm (which is generally much less responsive to Atropine) — the totally typical LBBB QRS morphology (as well as the heart rate of ~45-50/minute) suggest the escape rhythm in this case is more likely to be from the AV node, with LBBB rather than ventricular escape accounting for the QRS widening. For this patient with new-onset chest pain who is presenting within the first 6 hours of symptoms with obvious acute inferior STEMI — there is an excellent chance that Atropine might work. That said, the relatively modest ST-T wave depression in lead V1 (compared to V2) in the setting of acute inferior STEMI suggests likely acute RV involvement — and, this combination of factors suggests relative hypovolemia is likely to be an important component of this patient’s symptoms. Bottom Line: Sometimes, “Ya just gotta be there” — I’d probably try cautious fluid resuscitation initially while I was on the phone with my On Call Cardiologist urging immediate cath with hope that acute reperfusion might be the most effective approach. I might consider Atropine IF bradycardia worsened with associated hypotension if fluids didn’t work and cath lab activation was delayed …
Question #2 (by Rod) — relates to “Why” is there concordant ST elevation in leads V3,V4? Rod follows this by asking about “discordant” ST changes. Realizing how TRULY COMPLEX all of the ECG subtleties are in this case — the basics of what I would want in this case from my EMS providers are recognition of new-onset chest pain in a 66-year old with bradycardia and orthostatic hypotension, in association with an obvious acute STEMI based on the dramatic acute inferior ST elevation with reciprocal ST depression in leads I and aVL that we see here. I would be HAPPY if that was all my EMS team recognized — since this combination of clinical and ECG information is more than enough to justify expedited transfer to an Emergency facility with immediate cath and treatment capabilities. The overseeing ED physician and On Call Cardiologist can then attend to the important additional features of this case (ie, immediate trial of cautious fluids while expediting prompt cath lab assessment and treatment).
That said, in specific answer to Rod’s question — I’ll echo comments made earlier by Dr. Jones. Focus on confusing terms such as “appropriate” or “inappropriate” concordance or discordance in my opinion distracts from the main issues. The ST elevation that we see in leads V3 and V4 just should NOT be there … It is abnormal, and indicates acute infarction. We know this because of the associated ECG findings of dramatic inferior ST elevation, the obviously abnormal ST hyperacute changes in lead V5, and the reciprocal ST depression in leads I, aVL, V1,V2. In my opinion, there is NO place for “Sgarbossa criteria” in assessment of this tracing. Instead, the most likely explanation for the ST elevation in V3, V4 (put forth by Dr. Jones) — is that this patient has acute occlusion of a very dominant RCA that resulted in acute infero-postero STEMI + acute RV involvement, with additional ST elevation seen in leads V5,V6 due to large posterior branches (of the PDA) + supply (by this dominant RCA) with some additional coronary branches that extended to vascularize the anterior wall (seen as ST elevation in V3,V4). Focus on specific numeric Sgarbossa criteria without allowance for the complex interaction of the numerous factors operative here will miss the overall picture of what is going on. Perhaps this is why the obvious acute inferior stemi was initially missed …
Ken Grauer, MD www.kg-ekgpress.com [email protected]
Update
The patient was treated in the Emergency Dept with fentanyl, fluids and pacing, as her rate dropped to 38 with a BP of 78/38. She was taken to the cath lab within about 20 minutes. She was found to have a 100% occlusion of the proximal to mid right coronary artery. She also had a temporary pacemaker inserted. Her post-cath ECG showed no interventricular conduction delay. She did well, and was expected to be discharged home within a few days.
Dawn Altman, Admin
Thormbolysis
Hi...I want to ask about the management of same patient in a facility where PCI isn't available and transfer to nearest such facility will take 4hrs. Pt presents with similar ECG. Can we thormbolyse this patient? And does Q waves in inferior leads in this patient suggest that time to thrombolyse has passed.
And How should we interpret Q waves+ ST elevations with reciprocal leads ST depressions with respect to Thrombolysis? Plz kindly guide
Farhan
REPLY to Farhankhan re "Thrombolysis"
My THANKS to Farhankhan for this question regarding “Thrombolysis”. Although this case was first presented over 4 years ago (ie, on 6/28/2017) — your question remains timely today, as we enter 2022! The HISTORY is the 1st KEY part of the answer — This patient’s new-onset chest pain ONLY begain 4 hours before presentation — so that is acute, and this already suggests that with a new acute STEMI — acute reperfusion IS indeed indicated.
The Q waves in lead III and aVF not relevant to the decision regarding reperfusion therapy in this case — because it is common with LBBB to see inferior lead QS complexes. But even if there was not LBBB — Q waves alone are NOT a good indicator by themselves. The entire clinical situation needs to be considered. This is because Q waves could reflect old injury — with new acute STEMI superimposed. In addition, we now appreciate that although Q waves can serve as a longterm marker of prior infarction — significant Q waves CAN form acutely in as little as 1-to-2 hours! Thus regardless of Q waves — an acute history plus obviously acute ST-T wave changes provides ample indication for acute reperfusion.
BOTTOM LINE: The STEMI in this case is obviously acute. If cath facilities were not readily available — then thrombolysis is strongly advised (of course assuming no contraindications to thrombolysis).
Ken Grauer, MD www.kg-ekgpress.com [email protected]