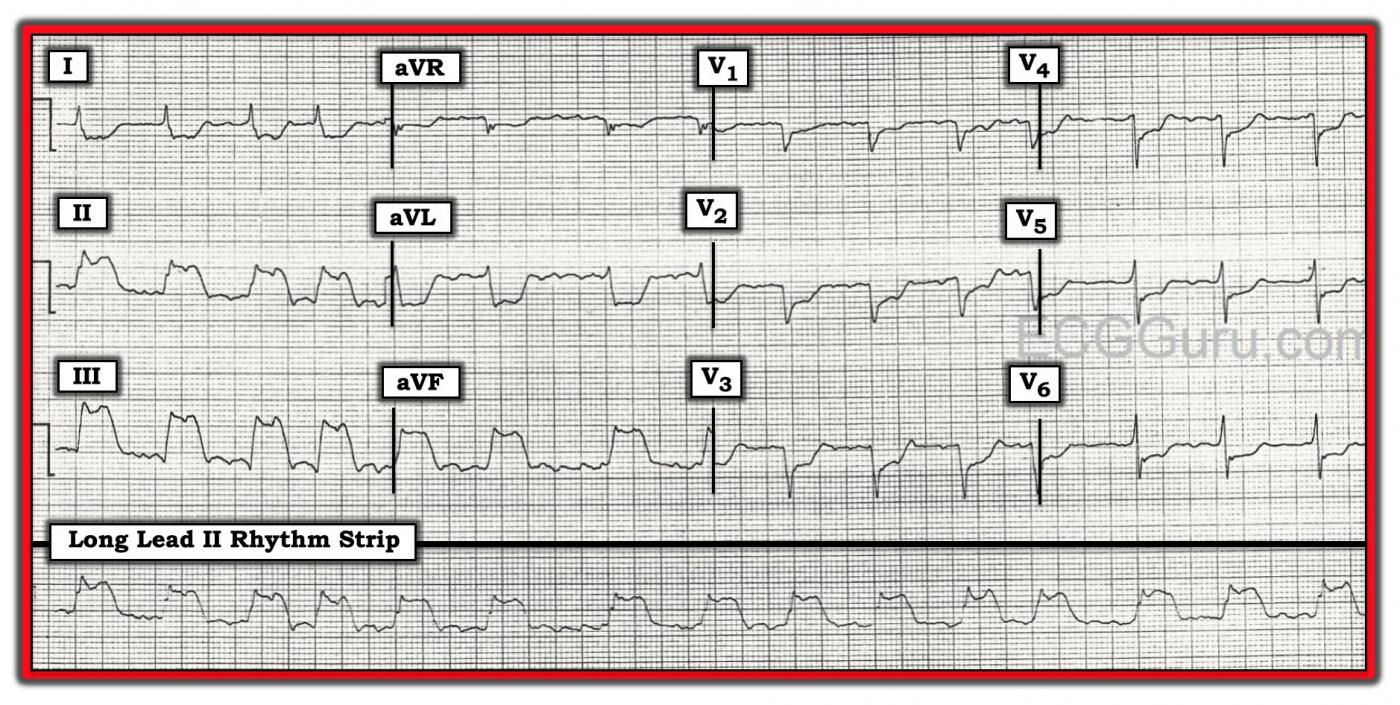
The Patient This ECG was obtained from a 74-year-old man who had a history of COPD. He was complaining of severe chest pain at the time of the ECG.
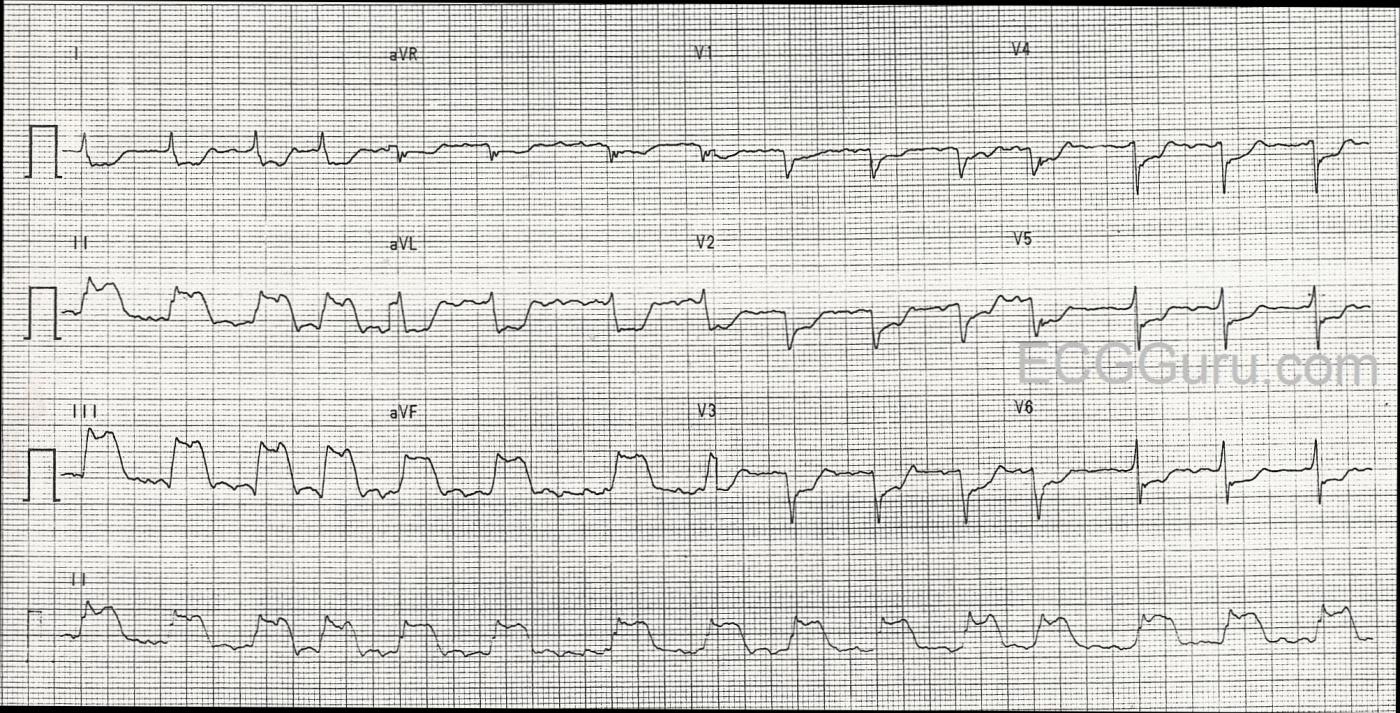
The ECG The rhythm is atrial fib or flutter (the R to R intervals are irregular, but seem to repeat about 4 interals). Flutter waves are seen during some of the longer intervals. The rate is approximately 90 beats per minute. The ST segments are very noticeably elevated in Leads II, III, and aVF. There is reciprocal ST depression in Leads I and aVL, and also in all the precordial leads.
There is poor R wave progression, with the transition from negative to positive in V5. The QRS width is difficult to determine with so much ST elevation and depression. Lead I and Leads V4 through V6 have fairly clear onset and offset (J point), and appear to be about .09-.10 seconds. There are possible pathological Q waves in the precordial leads: V1 through V4. If this represents old anterior wall M.I., that would explain the poor R wave progression.
Discussion This patient has definitely earned a trip to the cath lab. The ST elevations in related leads II, III, and aVF with expected reciprocal changes in Leads I and aVL are indicative of Inferior Wall ST elevation M.I. (STEMI). The rate is faster than we would like to see in an injured heart, and attempts will be made to slow it with medication. The ST depression in the precordial leads could have several causes, including subendocardial ischemia. It is common to see LOCALIZED ST depression in V1, V2, and V3 with inferior wall M.I., indicating extension of the damaged tissue up the posterior wall. The patient’s preexisting pulmonary disease could also be causing ST changes, as well as the poor R wave progression and the arrhythmia. Sadly, we do not have a previous ECG for comparison, or any information on the patient’s outcome.
For teaching purposes, this ECG is excellent for illustrating the difference between wide QRS and tall ST elevation. Since all four channels on this ECG are run simultaneously, we only have to compare the QRS complexes that look “wide” (II and III) to the complexes directly above (I), and we will see that the ST segment is masquerading as a wide QRS.
We are providing one image marked up in Dr. Ken Grauer’s signature style, and also a “clean” copy for those who wish to reproduce it without the labels.
All our content is FREE & COPYRIGHT FREE for non-commercial use
Please be courteous and leave any watermark or author attribution on content you reproduce.


Comments
AFib + Acute STEMI + Plus What Else?
The ECG in the Figure was obtained from a 74-year old man who presented with new-onset chest pain. Of note — the patient had a history of severe COPD? For teaching purposes — I’ll spotlight a number of interesting findings in the form of questions:
1) What is the rhythm?
2) What is the probable “culprit” artery of this STEMI?
3) Which areas of the heart are affected? (HINT: Your answer should list at least 3 different areas!).
4) Why do you think there is so much ST depression?
5) Is there any finding on this ECG that may be the result of this patient’s severe pulmonary disease?
=============================
1) The Rhythm: The rhythm is fast and irregular. It looks to be irregularly irregular (ie, the R-R interval changes from beat to beat). Although there do appear to be repetitive deflections in the baseline in a number of leads — to my measurement, these deflections are not nearly as regular and consistent as they should be if the rhythm was atrial flutter. Knowing that AFib may at times “organize” for brief periods of time (and therefore look a little like flutter periodically) — I would interpret this rhythm as AFib with a rapid ventricular response. As to width of the QRS complex, as per Dawn — despite looking wide in the inferior leads — we KNOW that the QRS complex is narrow from the fact that the other 9 leads on the tracing clearly show the QRS complex to be of normal duration.
2) The “Culprit” Artery for this STEMI? There is dramatic ST elevation in each of the inferior leads — with the amount of ST elevation maximal in lead III. In addition, there is reciprocal (ie, “mirror-image” ) ST depression in lead aVL, as well as in lead I. Thus, there is an acute inferior STEMI. Approximately 85% of inferior STEMI’s are the result of acute occlusion of the RCA (Right Coronary Artery), rather than the LCx (Left Circumflex Artery). The fact that ST elevation in lead III is more than in lead II — and that there is also dramatic mirror-image ST depression in lead aVL — both strongly suggest the RCA as the “culprit” artery.
3) Areas of the Heart Affected by this STEMI? In addition to acute inferior MI — ST depression in the anterior leads that is maximal in V2-V4 suggests associated acute posterior MI. Final support of the RCA as the probable “culprit” artery is forthcoming from the fact that the amount of ST depression in lead V1 is relatively modest compared to other anterior leads. This strongly suggests acute RV (right ventricular) MI — because lead V1 is also a “right-sided” lead, and with acute RV MI — some of the ST elevation from the RV is attenuated by the ST depression that would have been seen in V1 due to the posterior MI. The LCx artery does not supply the right ventricle — so evidence suggesting acute RV involvement tells us the RCA is the "culprit" artery. (NOTE: Right-sided leads would be needed to confirm acute RV MI — though this ECG is strongly suggestive of this! ).
4) Why So Much ST Depression? I strongly suspect this patient has multi-vessel disease! As per Dawn, there is a QS complex in leads V1 and V2. I think there is a small-but-present initial r wave in lead V4. It is more difficult to be certain about lead V3. This is because of the 3 beats present in lead V3 — a small initial r wave appears to be present for the middle beat, but not the two others. That said, this is really an academic point. The fact that we see the QS complexes that I note above, together with a disproportionate amount of ST depression in so many other leads (well beyond what should be expected for reciprocal changes and posterior infarction) — to me suggests high probability of prior anterior MI + diffuse subendocardial ischemia from severe multivessel coronary disease.
5) Any Sign on this ECG of Pulmonary Disease? One of the characteristics of patients with severe pulmonary disease — is persistence of S waves across the precordium. The finding of an otherwise unexplained, relatively deep S wave all the way through to lead V6 (as we see on this tracing) — is consistent with this patient’s known severe COPD. We’d need a baseline tracing in order to verify these persistent precordial lead S waves as a longstanding ECG finding.
=============================
FOR MORE on the ABOVE POINTS:
— What is the “Culprit” Artery? — CLICK HERE —
— ECG Findings with RVH / Pulmonary Disease — CLICK HERE —
Ken Grauer, MD www.kg-ekgpress.com [email protected]
Great Teaching Case but an Unfortunate Patient
Thanks to Dawn and Dr. Grauer for excellent observations on one of the worst ECGs one could ever see. So many problems on a number of levels! I'm not going to comment so much on what has already been noted but rather on a few more observations one should consider when presented with an ECG such as this one.
Diagnosing from a 12-lead ECG is always a look backward - you are determining what has already happened. Obviously, this is necessary because you must know the patient's current status. But true ECG interpretation also involves looking forward - using that information to anticipate what may happen in the future and, more specifically, what may happen in the next few hours or days.
First, the atrial fibrillation - if new - is very, very ominous. It implies a very proximal occlusion of the dominant RCA (in this case). The artery to the SA node (which, by the way, happens to be the main blood supply to the right atrium) is provided by the RCA in 55% of cases and the LCx in 45% of cases. This has nothing to do with vascular dominance; a non-dominant LCx could still be the source of the artery to the SA node. Of course, the RV infarction also implies a proximal RCA occlusion, but not necessarily as proximal as an occlusion of the SA nodal artery which is always the first or second branch of the RCA very close to its origin from the aorta. The SA node does not have the dual circulation that the AV node enjoys, so its involvement is usually permanent and devasting. Not only is the SA node wiped out but also a good bit of the right atrium as well. The appearance of atrial fibrillation during an acute MI carries a worse prognosis than ventricular fibrillation occuring during the acute phase of an MI.
I certainly agree that there appears to have been a remote anterior MI due to the initial QRS changes in V1 and V2. And this presents another ominous issue: the blood supply to the septum comes from the LAD and the RCA. Usually one protects the septum from an occlusion by the other but now we've lost both sources of circulation. This is a perfect setup for a septal rupture which sometimes occurs 2-4 days after the second infarction. The physicians and nurses caring for this patient (assuming he survived) should be aware of this.
Right ventricular infarctions almost never occur in a "normal" right ventricle - there is almost always some degree of RVH present. That's why I found Dr. Grauer's comment about pulmonary changes on the ECG so interesting: I think it is quite possible that this patient has a Type C right ventricular hypertrophy which would account for the RV infarction and the deep S waves across the precordium. Type C RVH is unusual because it does not manifest tall R waves in V1 or V2 (often no R waves at all!) but it is characterized by deep S waves across the precordium. It is a result of advanced COPD.
Thanks for a great website, Dawn. And thanks to Dr. Grauer for your insights (and for a great website also!).
Jerry W. Jones MD FACEP FAAEM
https://www.medicusofhouston.com
Twitter: @jwjmd
THANK YOU Dr. Jones !!!!
Hi Jerry. Absolutely SUPERB comments you make here — THANK YOU! — :) Ken
Ken Grauer, MD www.kg-ekgpress.com [email protected]